
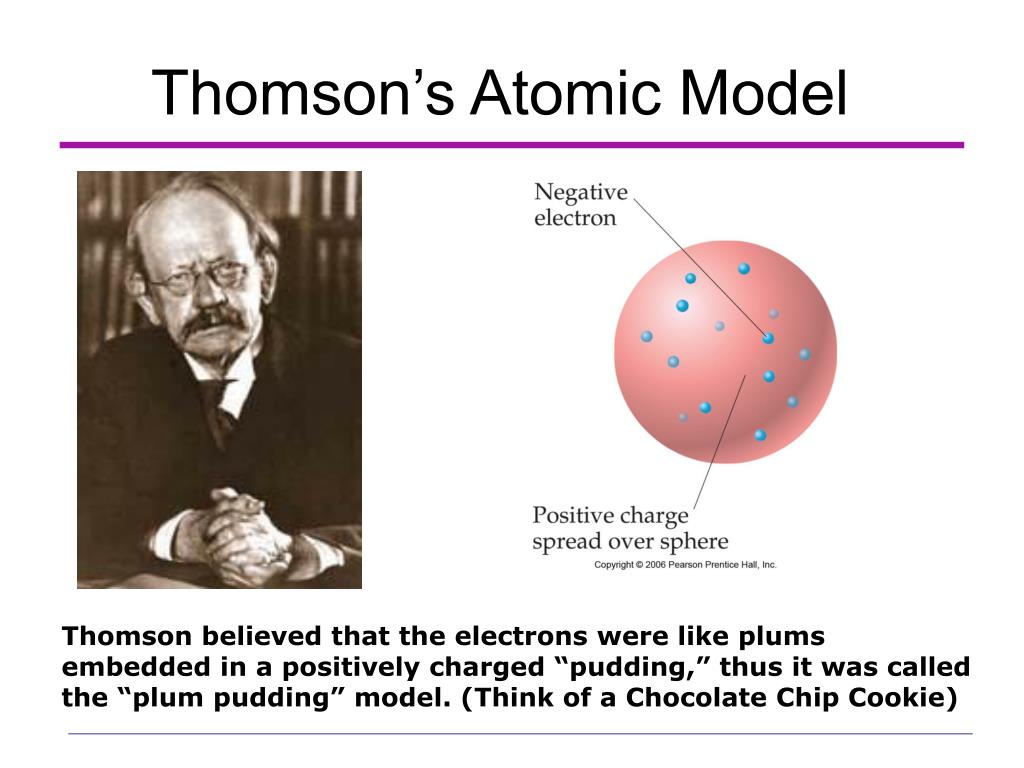
In 1913 Thomson published an influential monograph urging chemists to use the mass spectrograph in their analyses. His nonmathematical atomic theory-unlike early quantum theory-could also be used to account for chemical bonding and molecular structure (see Gilbert Newton Lewis and Irving Langmuir). Of all the physicists associated with determining the structure of the atom, Thomson remained most closely aligned to the chemical community. Only some of the s clearly defined atoms had much. But Thomsons atom model failed to explain Rutherfords -particle scattering experiment in which most of the fast-moving - particles went through the gold foil straight away. He was a good lecturer, encouraged his students, and devoted considerable attention to the wider problems of science teaching at university and secondary levels. Limitations of JJ Thomson Model of Atom (Plum Pudding Model) In magnitude the whole atom was electrically neutral. Even though he was clumsy with his hands, he had a genius for designing apparatus and diagnosing its problems. In 1884 he was named to the prestigious Cavendish Professorship of Experimental Physics at Cambridge, although he had personally done very little experimental work. This cylinder had two slits in it, leading to electrometers, which could measure. His first experiment was to build a cathode ray tube with a metal cylinder on the end. He was then recommended to Trinity College, Cambridge, where he became a mathematical physicist. Thomson had an inkling that the ‘rays’ emitted from the electron gun were inseparable from the latent charge, and decided to try and prove this by using a magnetic field. In 1923, Millikan won the Nobel Prize in physics in part because of this experiment. George FitzGerald and Walter Kaufmann found similar results. Thomson discovered negatively charged 'corpuscles' with a mass about 1,840 times smaller than that of a hydrogen atom. Instead young Thomson attended Owens College, Manchester, which had an excellent science faculty. Experimenting with cathode rays in 1897, J. His father intended him to be an engineer, which in those days required an apprenticeship, but his family could not raise the necessary fee. Ironically, Thomson-great scientist and physics mentor-became a physicist by default. We strive for accuracy and fairness.From "The Growth of Physical Science," by Sir James Hopwood Jeans (Cambridge: Cambridge University Press, 1948) Early Life and Education He died in Cambridge on August 30, 1940, and is buried in Westminster Abbey near two other influential scientists: Isaac Newton and Charles Darwin. He left research in 1918 to become Master of Trinity College. In addition to being awarded the Nobel Prize in 1906, he was knighted in 1908 by King Edward VII.

Thomson published 13 books and more than 200 papers in his lifetime. They had one daughter, Joan, and one son, George Paget Thomson, who went on to become a physicist and win a Nobel Prize of his own. Thomson married Rose Paget, one of his students, in 1890.
This was the first use of mass spectrometry. In doing so, he discovered that neon was composed of two different kinds of atoms, and proved the existence of isotopes in a stable element. This led to one of his other famous discoveries in 1912 when he channeled a stream of ionized neon through a magnetic and an electric field and used deflection techniques to measure the charge to mass ratio. In 1906, Thomson began studying positively charged ions, or positive rays.


 0 kommentar(er)
0 kommentar(er)
